EXPERINMENT 08
Object
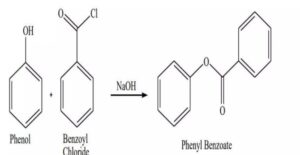
To prepare Phenyl benzoate from phenol
Reference
- Peesapati venkateshwarlu, Elementary practical organic chemistry, 2nd edition, 1966, published by CBS publisher, page No. 172
Requirement
Iodine flask, beaker, measuring cylinder, pipette
chemical:
- Benzoyl chloride 2ml
- Phenol 1gram/ 1ml
- NaOH solution 10%w/v
- ethyle alcohol
Theory.
Phenyl benzoate is synthesized by reacting phenol with benzoyl chloride in the presence of a base like sodium hydroxide, utilizing the Schotten-Baumann reaction. This method leverages the nucleophilic nature of the phenoxide ion, formed when phenol reacts with the base, to attack the electrophilic carbonyl carbon of benzoyl chloride, resulting in the formation of the phenyl benzoate ester.
Phenol (C6H5OH) reacts with sodium hydroxide (NaOH) to form a phenoxide ion (C6H5O-) and water. This reaction is facilitated by the strong base, which deprotonates the phenol, making the oxygen atom a better nucleophile.
- The phenoxide ion, with its negative charge, acts as a nucleophile and attacks the electrophilic carbonyl carbon of benzoyl chloride (C6H5COCl).
Formation of the Ester:
- This attack leads to the displacement of the chloride ion (Cl-) from benzoyl chloride, forming phenyl benzoate (C6H5OOCC6H5) and hydrochloric acid (HCl).
Schotten-Baumann Method:
The reaction is typically carried out under basic conditions (using sodium hydroxide) and with vigorous shaking to ensure good mixing and reaction progress.
The phenyl benzoate, being insoluble in water, precipitates out as a solid, allowing for easy separation from the aqueous solution.
Procedure
- 1 gram of phenol is dissolved in 15 mL of 10% sodium hydroxide solution in an Erlenmeyer flask.
- 2 mL of benzoyl chloride is added.
- Close the mouth of the flask with a rubber stopper and shake vigorously for about 30 minutes.
- filter the crystal and recrystalize it with ethyle alcohol.
Result
Phenyl benzoate prepared from phenol
