Object : To Prepare picric acid from phenol
Reference
- Agarwal O.P., Practical Organic chemistry, published by Krishna educational publisher, Edition 2014, page no. 316
- Mann F.G. , Saunders B.C. , “practical organic chemistry, published by orient hangmen private ltd., 4th edition, page N. 173
Requirement:
Glassware : Conical Flask, Glass rod, Measuring Cylinder, Pipette
Chemicals : Phenol, Con. H2SO4, Con. HNO3
Theory :
Picric acid is 2,4,6-trinitro-phenol. It is yellow in colour. It is used as tropical anti infective and disinfectant so that used as a cleaning agent and also preservative due to presence of phenol. Picric acid is obtained by nitrating phenol.
The nitration of aromatic compounds is usually done by using conc. HNO3 in presence of conc. H2SO4. Nitration
of aromatic compounds is an example of electrophillic aromatic substitution.
Nitration is usually carried out at low temperature. At high temperature there is loss of material due to oxidation by HNO3. Phenol being an activated nucleus towards electrophillic aromatic substitution, the nitration reaction occurs very easily. It undergoes nitration with HNO3 even at room temperature forming ortho & para nitrophenol which can be separated by steam distillation. Phenol when treated with conc. HNO3 in presence of conc. H2SO4 undergoes nitration at both ortho and para position to yield picric acid.
Chemical Reaction
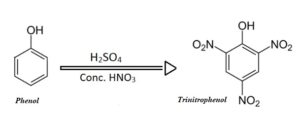
Procedure
- Place 4gm of phenol to conical flask and add 5ml of Con. H2SO4
- mix the solution properly (solution become warm)
- heat the flask on water bath for 30 minutes.
- After 30 minutes place the flask on Ice bath.
- Now add 20 ml of Con. HNO3 & shake it properly
- Mixture allow to stand undisturbed until red fumes stops poring out.
- when fumes stop, heat the flask on water bath for 1-2 hrs with occassional stirring.
- after given time add 100 ml of cold water and chill the mixture.
- filter the yellow crystals
- wash with water to remove excess of inorganic acids.
Observation
Yellow colored crystal are obtained
Result
Picric acid was prepared successfully
